
Snapshot
- Sugar the secret to lasting battery tech
- Lithium-sulfur promises lower production costs, lighter weight
- Enserv Australia aims to have batteries in production soon
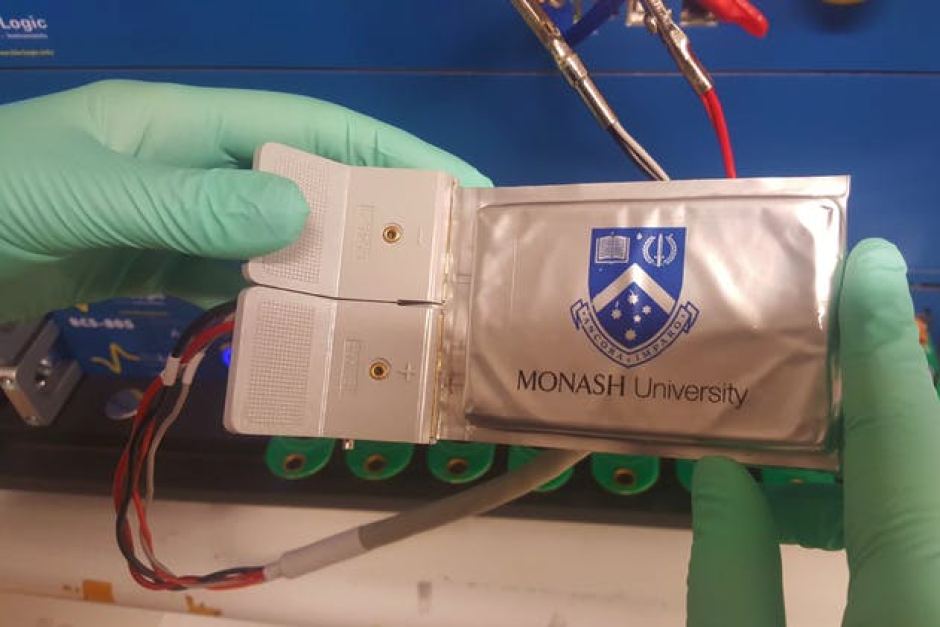
A research team at Monash University has come up with, as they put it, a “saccharide-based binder for efficient polysulfide regulations in Li-S batteries”. For us laypeople, they’re adding sugar to the battery to increase its durability and potentially make lithium-sulfur batteries a contender for transport applications.
Inspired by a 1988 geochemistry report that experimented with mixing glucose and cellulose to resist degradation in sulfur-based mixtures, Professor Mainak Majumder and his team were able to develop robust cathodes (the bit of the battery energy passes through) that can survive at least 1000 charge-discharge cycles (how many times you deplete to 0 per cent then recharge the battery to 100 per cent) and still provide a large amount of energy.
This is a significant milestone to creating a commercially viable lithium-sulfur battery – something that currently does not exist.
LFP (iron) and NMC (nickel manganese cobalt) lithium-based batteries are the main battery technology in electric cars today, but lithium-sulfur-based batteries have shown promise as a rechargeable battery that can be extremely light with a lower manufacturing cost.
Any battery nerd reading this might think 1000 cycles isn’t so impressive – and they’d be right – but until now, lithium-sulfur batteries were largely ignored as a viable alternative because they couldn’t survive more than 50-or-so charge cycles until they no longer provide a usable amount of energy.
Lithium iron phosphate batteries (LFP) can withstand over 3000 charging cycles before capacity is lost. Great stuff, but that durability comes with a major weight penalty.
Theoretically, a lithium-sulfur battery can store 400-600 watt hours of energy per kilogram, a massive increase over the 125-160 watt hours per kilogram found in typical electric car batteries now.
Using a lithium-sulfur battery pack of the same weight and size, an electric car could travel three times as far (or further) when compared to the lithium iron phosphate or lithium nickel manganese cobalt technology used today.
In practice, a Tesla Model 3 Standard Range Plus, which has an approximately 500kg battery that provides 400km of real-world range, could end up with a battery the same size and weight but with a whopping 1200km of range.
Alternatively, Tesla could drop 250kg of weight from the vehicle with a battery half the size, resulting in cost savings, and still provide a very usable 600km of range.

The research carried out by the Monash team means not only could it be possible to develop a lithium-sulfur battery with all the good things they bring – like two or three times the energy for the same size and weight, with a cheaper and more environmentally-friendly manufacturing program – but also with the same charging durability we’ve come to expect from existing electric vehicle batteries.
The Monash team currently has a prototype pouch battery with an energy density of 206 watt hours per kilogram, but Enserv Australia plans to develop the technology further and manufacture lithium-sulfur batteries in Australia within the next five years.
Batteries are one of those areas of research that someone, somewhere, claims to have found a breakthrough with almost daily, so take this one with a grain of salt (or sugar) as to when we’ll see this technology on the road.
The full details of Monash University’s research can be found in the Nature Communications journal.




